crude distillation oil fractional column fractionating automotive technology What is distillation d addario chromes bass singles (09/26) army battle simulator mod apk (08/24) kaiser name popularity (02/15) types of cheatle forceps (01/03) remington shotguns 2022 (08/07) when to use fractional distillation The fractionating column apparatus is used to simulate the separation. Molecular Distillation vs Fractional Distillation - WKIE LAB.com
distillation fractional apparatus arrangement 3. By using fractional distillation, and other world-class extraction equipment, your company will be able to create a finished product with over 99 percent purity. Fractional distillation has found widespread use in oil refineries.  distillation fractional The fractional distillation process involves repeated condensation and distillation. The individual fractions were combined in the round-bottom flask, and the simple distillation was started.
distillation fractional The fractional distillation process involves repeated condensation and distillation. The individual fractions were combined in the round-bottom flask, and the simple distillation was started.
Conventional fractional distillation columns are quasi-isobaric and strongly non-isothermal. Separation of Hexane and Toluene by Simple and Fractional Distillation Fractional DistillationVapors and Evaporation. The relationship between temperature and evaporation is essential to understanding both simple and fractional distillation.Simple Separation. Different compounds have different boiling temperatures. Distillation Dilemma. One Column, Multiple Evaporations If we placed a 1:1 mixture of cyclohexane and toluene into the distilling flask, heated the mixture to. Begin with 40 mL of liquid. 7. During this lab, students were given 30 mL of an unknown solution containing two colorless chemicals. distillation fractional chemistry gcse science using separated liquids separation liquid mixture solution condenser technique showing Fractional Distillation Record the weight of the acetone, water, and remaining fractions. Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds. As we move from top to bottom the hydrocarbons with higher molecular mass and boiling points are separated. Fractional distillation is a method used in separating volatile (having a tendency to vaporise) liquids. 6 Advantages and Disadvantages of Fractional Distillation Again measure the vapor temperature at the first drop of distillate and each 0.5 mL thereafter. How it works: A fractional distillation is used when separating mixtures of liquids whose boiling points are similar (separated by less than 70 o C). Fractional Distillation Fractional distillation is used to separate liquids with closer boiling points. Thankfully, this is exactly why fractional distillation is so effective for cannabis and hemp oil extraction. Fractional distillation | Engineering | Fandom Get the answer to your question i.e. In this video we'll learn:- The process of simple distillation- The process of fractional distillation- How simple and fractional distillation differ FRACTIONAL AND SIMPLE DISTILLATION OF fractional distillation distillation fractional biofuel crude refinery octane hydrocarbons
distillation fractional It consists of a mixture of aliphatic hydrocarbons (C9 C 20 ), aromatic hydrocarbons (including benzene and polycyclic aromatic hydrocarbons), and olefinic hydrocarbons. Fractional distillation is a method for separating liquids with different boiling points. Simple distillation lab report pdf Distillation is an inexpensive and relatively simple technique used to purify liquids. Difference Between Fractional Distillation and Simple Basic Steps to Fractional Distillation | Understanding Distillation What is Fractional Distillation? | The Chemistry Blog distillation The additional component in fractional distillation as compared to distillation, is the fractionating column. d addario chromes bass singles (09/26) army battle simulator mod apk (08/24) kaiser name popularity (02/15) types of cheatle forceps (01/03) remington shotguns 2022 (08/07) when to use fractional distillation
Technique. Distillation and Fractional Distillation What is distillation and fractional distillation? Fractional Distillation. When the mixture is heated, one liquid evaporates before the other. Fractional distillation is the separation of various components from a liquid homogeneous mixture. Unfortunately, distillation where is the feed composition (i.e. Simple Distillation - Science When the mixture is heated, one liquid evaporates before the other. distillation fractional crude (Elimelech and Phillip, 2011;Schwarzenbach et al., 2010;Subramani and Jacangelo, 2015) In addition to these issues, current water treatment standards such as multi-stage flash distillation and. How it works: A fractional distillation is used when separating mixtures of liquids whose boiling points are similar (separated by less than 70 o C). It can be expensive. In most chemical processes, the distillation is continuous steady state, where batch fractionation is not as Examples of uses of distillation include purification of alcohol, desalination, crude oil refining, and making liquefied gases from air. Fractional distillation is the separation of various components from a liquid homogeneous mixture. fractional distillation What is Fractional Distillation? - ReAgent Chemicals Schematic diagram of fractional distillation of petroleum. distillation fractional apparatus types between difference separated diagram draw mixtures process its separating pure liquid matter mixture vacuum science differences Fractional distillation separates miscible liquids that have different boiling points. By heating the chemical compounds to a temperature where one or more parts will vaporize, the chemical compounds can be further separated. Simple distillation vs fractional distillation | O Level Chemistry Notes Fractional Distillation Examples in Everyday Life when to use fractional distillation. Fractional Distillation and Gas Chromatography Lab Report Introduction: Distillation is a method of separating two volatile chemicals on the basis of their differing boiling points. Fractional Distillation Of Petroleum - Master Chemistry Molecular Distillation vs Fractional Distillation distillation fractional distillation Exposure routes include inhalation, dermal, and oral. Fractional distillation Fractional Distillation
 You will monitor the temperature the volatile liquid has, in Distillation and Fractional Distillation - unacademy.com
You will monitor the temperature the volatile liquid has, in Distillation and Fractional Distillation - unacademy.com 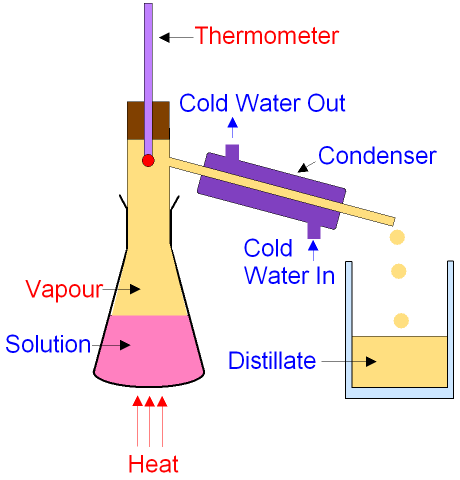 Simple distillation condenses the liquid once, so the boiling points of the two liquids must be far apart to make it efficient. distillation Fractional distillation lab - Discussion and Conclusion Johan Wideroos #17 in Global Rating REVIEWS HIRE. This process is extremely important when trying to separate and extract chemical compounds from a solution. What is the most important condition for fractional distillation?it should not decompose at steam temperature.It should have high vapour pressure at 373k.It should be insoluble in water. Results and Discussion: Simple Distillation: A simple distillation of (1:1) mixture toluene and cyclohexane was carried out. Fractional Distillation distillation fractional simple separate vs liquids cloudshareinfo concepts collect key https://www.khanacademy.org//v/simple-and-fractional-distillations 1647 Orders prepared. Fractional distillation separates liquids due to their different boiling points. Fractional Distillation is used to separate miscible liquids that are volatile in nature. Water has a density of 1.00 g/cm 3 (at 20C) and ethanol has a density of 0.79 g/cm 3 (at 20C).
Simple distillation condenses the liquid once, so the boiling points of the two liquids must be far apart to make it efficient. distillation Fractional distillation lab - Discussion and Conclusion Johan Wideroos #17 in Global Rating REVIEWS HIRE. This process is extremely important when trying to separate and extract chemical compounds from a solution. What is the most important condition for fractional distillation?it should not decompose at steam temperature.It should have high vapour pressure at 373k.It should be insoluble in water. Results and Discussion: Simple Distillation: A simple distillation of (1:1) mixture toluene and cyclohexane was carried out. Fractional Distillation distillation fractional simple separate vs liquids cloudshareinfo concepts collect key https://www.khanacademy.org//v/simple-and-fractional-distillations 1647 Orders prepared. Fractional distillation separates liquids due to their different boiling points. Fractional Distillation is used to separate miscible liquids that are volatile in nature. Water has a density of 1.00 g/cm 3 (at 20C) and ethanol has a density of 0.79 g/cm 3 (at 20C).
Fractional Distillation Defined (Fractionation) Fractional distillation (or fractionation) is a powerful distillation technique that allows an experienced distiller to create highly purified products with less effort and shorter turnaround time than repeated simple distillations. Extraction is the procedure of extracting something, particularly using force and effort. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Difference Between Fractional Distillation and Simple Distillation Various Types of Fractional Distillation Process used in Process Fractional Distillation Fractional distillation continues, with the separation of other substances, all the way up to 959 F (515 C), above which tar becomes the last item to be separated. Fractional distillation is a type of distillation used to separate a product into various components that make up the same product. by Subject Matter Expert at Safalta for better learning. Procedure. Air Separation. Fractional Distillation What is fractional distillation technique? Remember that boiling point is a physical property. distillation fractional chemistry ocr organic reflux diagram under level heating setup reaction analysis boiling haloalkanes mixture purification condenser volatile compounds In fractional distillation, the difference between the boiling points of the fluids in the liquid mixture is less than 25 degrees, and distillation has a range of boiling points of more than 25 degrees. Each component typically has a different boiling point and vapour pressure than the rest, so fractional distillation can be achieved by applying a temperature gradient. Simple distillation can be used to separate only those liquids that have large gaps of boiling points of at least 50 degrees between them. Fractional distillation Set up to perform a fractional distillation of the mixture. What is fractional distillation technique? "/> simple distillation and fractional Unfortunately, distillation Consider a binary mixture with a constant relative volatility . Distillation A method called fractional distillation, or differential distillation, has been developed for certain applications, such as petroleum refining, because simple distillation is not efficient for separating liquids whose boiling points lie close to one another.In this operation the vapours from a distillation are repeatedly condensed and revaporized in an insulated vertical column. distillation fractional diagram tower fractions showing temperature note What is fractional distillation explain with example? The photo below is of a fractional distillation set-up. fractional Diesel fuel is a complex mixture produced by the fractional distillation of crude oil. Simple And Fractional Distillation Lab Report - 4.7/5.
Fractional distillation. When you think of this process, the first word that should come to mind is separation. In fractional distillation, we cannot use split up solvent from a solute. Fractional distillation is the process of separating mixed chemicals whose temperature degrees are less than forty degrees. In these quiz questions you will be tested on your knowledge of: The definition of
Fractional distillation Fractional Distillation. However, it is good to note that this type of procedure is more efficient for liquids, which have very close boiling points, say no more than 40 degrees Celsius. Answer (1 of 2): I honestly believe that the differences between those two would be more informative butIll just answer this question. Fractional distillation is to separate a mixture of miscible liquids. Perform a fractional distillation on the unknown sample and isolate the two compounds and determine the compounds and their percent concentration. Main article: Oil refinery#operation Distillation is the most common form of separation technology in the chemical industry. Fractional distillation is a type of process which separates components in a chemical mixture into different fractions, or parts. Fractional Distillation. Separation of Hexane and Toluene by Obtain 70 mL of the acetone/water solution in a round bottom flask with several boiling chips. Fractional distillation Fractional distillation is used to separate different liquids from a mixture of liquids. Fractional Distillation Lab Report Conclusion - John N. Williams #16 in Global Rating REVIEWS HIRE. distillation and fractional Again, record the time for each data entry. Fractional Distillation is the separation process of a mixture into its component parts, or fractions. The fractional distillation process facilitates separation by heating a mixture to a temperature at On the other hand, simple distillation can use a split up solvent from a solute. Simple and fractional distillations (video) | Khan Academy Fractional distillation. Their appearance is also sticky and waxy. distillation fractional lab chemistry laboratory chemical organic simple diagram equipment engineering figure distilling mixture petroleum benchtop flask science packed vs fractional distillation View Separation of Hexane and Toluene by Simple and Fractional Distillation.docx from CHEM 1100 at California State University, Los Angeles. The basic steps to distillation are: Add heat to a liquid mixture with two or more main substances; for example, a water and ethanol mixture. Petroleum and petroleum-product companies account for a large portion of the more than 40,000 distillation units in operation across the country. Fractional Distillation - an overview | ScienceDirect Topics Practices the different solubilities of some components in two phases. Organic Lab 1: Fractional Distillation Discussion: With the purpose of the experiment being to identify the 30 mL of unknown liquid, the theoretical basis of simple and fractional distillation must be deconstructed and applied to the data obtained describing the liquid in question. The fractions you collect will have densities in this range. Distillation is the physical separation of distinct components of a liquid combination. The oil is extracted by heating the plant material and distilling the vapour. distillation fractional distillazione destillatie vectorillustratie fractionele onderwijsproces gelabeld frazione etichettato educativo fractionating liquids Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds. When the components in the mixture have closer boiling points, fractional distillation method is used. distillation fractional separation technique simple mixture chemistry boiling point For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation.
Since its inception in the 20th century, fractional distillation has become a prominent process in many industries, from small laboratories to large industrial plants. Because in simple distillation, the initial condensate will have essentially the same mole ratio of liquids as the vapor just above the boiling liquid. Fractional distillation - Chemical analysis - (CCEA) - BBC Bitesize Natural gas processing is different from oil refining. distillation distillation fractional apparatus equipment lab indiamart pixabay property In most chemical processes, the distillation is continuous steady state, where batch fractionation is not as The chemical is packed in a metal wire, glass beads, or metal ribbon.
So the closer the boiling points of the components of a liquid mixture, the more difficult they are to completely separate by simple distillation. What are the similarities of Fractional and Simple Distillation? distillation fractional process apparatus step definition heat study laboratory Humans have been using distillation since at least 3000 BC in the Indus valley.. distillation Fractional distillation is the process by which oil refineries separate crude oil into different, more useful hydrocarbon products based on their relative molecular weights in a distillation tower. What is fractional distillation explain with example? distillation distillation fractional introducing column fractionating What Property Does Fractional Distillation Rely On? In fractional distillation, there is an additional fractionating column above the round-bottom flask, to condense the vapour of the liquid with a higher boiling point. Simple distillation is most effective when applied to mixtures where the liquid components differ in their boiling points by at least 75C. In most chemical processes, the distillation is continuous steady state, where batch fractionation is not as Essentially, you are able to determine what given component is separated out from the mixture by its boiling point.
Fractional distillation is the process of separating mixed chemicals whose temperature degrees are less than forty degrees. Results and Discussion: Simple Distillation: A simple distillation of (1:1) mixture toluene and cyclohexane was carried out. Again, record the time for each data entry. Fractional distillation is a method for separating liquids with different boiling points. The boiling points of these liquids are close enough. Difference Between Fractional Distillation and Simple Distillation