 Neoleukin is a biopharmaceutical company creating next generation immunotherapies for cancer, inflammation and autoimmunity using de novo protein design technology. According to the preclinical data, NL-201 showed the ability to elicit and expand CD8+ and NK cells at low doses with reduced impact on immunosuppressive regulatory T cells.
Neoleukin is a biopharmaceutical company creating next generation immunotherapies for cancer, inflammation and autoimmunity using de novo protein design technology. According to the preclinical data, NL-201 showed the ability to elicit and expand CD8+ and NK cells at low doses with reduced impact on immunosuppressive regulatory T cells. Experimental results indicate that anti-myeloma activity is mediated by expansion of cytotoxic memory CD8 T cells and a decrease in T-regulatory CD4 cells in the bone marrow. In November 2021, Neoleukin announced the presentation of four abstracts highlighting new preclinical data on NL-201 at the Society for Immunotherapy of Cancers 36TH Annual Meeting (SITC 2021). New data demonstrated that NL-201 can activate the tumor microenvironment and increase T-cell receptor diversity in preclinical models. We expect to initiate a Phase 1 trial in 2022 to evaluate NL-201 in patients with these indications., Abstract number: 1609The IL-2/IL-15 Mimetic NL-201 Prevents Myeloma Relapse after ASCT by Expanding Highly Cytolytic T Cells in the Bone Marrow that are Resistant to Exhaustion, Abstract number: 4560NL-201, a De Novo Agonist of IL-2 and IL-15 Receptors, Demonstrates Synergistic Antitumor Activity with Anti-PD-1 Checkpoint Inhibitor Therapy in a Preclinical Non-Hodgkin Lymphoma Model, The ASH poster and abstract link are available on the Neoleukin website publications page:https://www.neoleukin.com/science/#pubs.
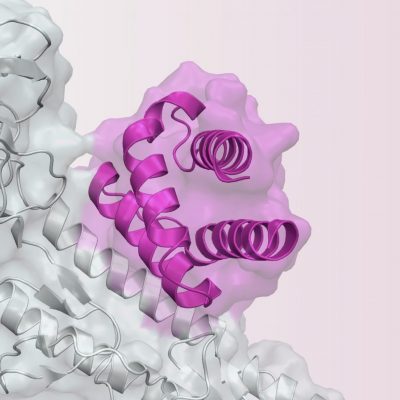
SEATTLE, Dec. 11, 2021 (GLOBE NEWSWIRE) -- Neoleukin Therapeutics, Inc., Neoleukin (NASDAQ:NLTX), a biopharmaceutical company utilizing sophisticated computational methods to designde novoprotein therapeutics, today announced the presentation of preclinical data on NL-201 in multiple myeloma at the 63rdAmerican Society of Hematology (ASH) Annual Meeting and Exposition taking place virtually and in person December 11-14, 2021. Mr. Palekar has served as the CEO of 89bio since June 2018. Please remove one or more studies before adding more. Furthermore, NL-201 treated mice had an increase in bone marrow T-cells expressing granzyme B and a decrease in the T-cell exhaustion phenotype. We believe it is the first fully de novo protein to enter clinical development, said Jonathan Drachman, M.D., Chief Executive Officer of Neoleukin. *KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Further information on potential risk factors that could affect Neoleukins business and its financial results are detailed under the heading Risk Factors in documents the company files from time to time with theSecurities and Exchange Commission(SEC), and other reports as filed with theSEC. Neoleukin Therapeutics has dosed the first subject with its NL-201 plus Keytruda (pembrolizumab), in a combination arm of Phase I clinical trial on relapsed or refractory solid tumour patients. Details as follows: Time: 1:30 p.m. Pacific / 4:30 p.m. Eastern, Webcast URL: http://investor.neoleukin.com/events. The trial will assess safety, pharmacokinetics, pharmacodynamics, immunogenicity, and antitumor activity. The Phase 1 study will be conducted at multiple sites in Australia and North America. Further information on potential risk factors that could affect Neoleukins business and its financial results are detailed under the heading Risk Factors in documents the company files from time to time with the Securities and Exchange Commission (SEC), and other reports as filed with the SEC. The Phase 1 study is planned to enroll up to 120 patients with advanced, relapsed, or refractory solid tumors. Contract Research Organisation for Clinical Trials, Thank you for subscribing to Clinical Trials Arena, Hard data and deep insights on clinical trials strategy & operations, Receive our newsletter - data, insights and analysis delivered to you. The gain of $7.8 million recognized was the total consideration of $8.2 million, less transaction costs of $0.4 million. Safety follow- up will occur within 7 days after the last dose of investigational product. Neoleukin uses sophisticated computational methods to design proteins that demonstrate specific pharmaceutical properties that provide potentially superior therapeutic benefit over native proteins. Additionally, a published abstract in Blood reported on NL-201 antitumor activity in preclinical studies of non-Hodgkin lymphoma. Our progression to a clinical stage company is a significant milestone, and we remain focused on execution of our clinical development strategy and pipeline expansion as we advance and explore the potential of our de novo protein technology platform, said Jonathan Drachman, M.D., Chief Executive Officer of Neoleukin. Neoleukin undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. The increase was primarily due to increased expenses incurred from IND-enabling and clinical trial activities related to Neoleukin's lead product candidate, NL-201, and in connection with the advancement of other Neoleukin technologies. Individual Participant Data (IPD) Sharing Statement: Studies a U.S. FDA-regulated Drug Product: Studies a U.S. FDA-regulated Device Product: Recommended phase 2 dose (RP2D) for NL-201 (Parts 1 and 2) [TimeFrame:Up to Day 33], Recommended dose schedule for NL-201 (Parts 1 and 2) [TimeFrame:Up to Day 33], Recommended phase 2 dose (RP2D) for NL-201 in combination with Pembrolizumab (Parts 3 and 4) [TimeFrame:Up to Day 33], Recommended dose schedule for NL-201 in in combination with Pembrolizumab (Parts 3 and 4) [TimeFrame:Up to Day 33], Incidence of treatment-emergent adverse events [TimeFrame:Up to Day 33], Severity of treatment-emergent adverse events [TimeFrame:Up to Day 33], Best Objective Response according to RECIST version 1.1 [TimeFrame:Up to 36 months], Objective Response Rate (ORR) according to RECIST version 1.1 [TimeFrame:Up to 36 months], Progression-Free Survival (PFS) according to RECIST version 1.1 [TimeFrame:Up to 36 months], Duration of Response (DOR) according to RECIST version 1.1 [TimeFrame:Upto 36 months], Pharmacokinetic (PK) profile of NL-201 by half-life (t1/2) [TimeFrame:Up to 24 Months], Pharmacokinetic (PK) profile of NL-201 by area under the plasma concentration time curve (AUC) [TimeFrame:Up to 24 months], Pharmacokinetic (PK) profile of NL-201 by maximum observed plasma concentration (Cmax) [TimeFrame:Up to 24 months], Pharmacokinetic (PK) profile of NL-201 by volume of distribution (Vd) [TimeFrame:Up to 24 Months], Immunogenicity of NL-201 [TimeFrame:Up to 24 months], Flow cytometry analysis of immune cells in blood [TimeFrame:Up to 36 months], Serum measurements of inflammatory cytokine levels [TimeFrame:Up to 36 months], Analysis of immune characteristics of the tumor microenvironment [TimeFrame:Up to 36 months], Estimate additional measures of anti-tumor activity of NL- 201 per iRECIST criteria [TimeFrame:Up to 36 months], Patients with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, At least 6 weeks from any prior nitrosurea or mitomycin C therapy; at least 4 weeks from any other prior chemotherapy or checkpoint inhibitor; at least 2 weeks from any kinase inhibitor, Part 1 Only: Patients with relapsed or refractory advanced solid tumor, other than prostate cancer, who have progressed, not tolerated or are ineligible for all approved lines of therapy, Part 2 Only: Patients with kidney and skin cancer who have failed at least 1 line of systemic therapy, Part 3 Only: Patients with solid tumors who have received 1 prior line of therapy for advanced or metastatic disease, Part 4 Only: Patients with diagnosed target disease OR previously received pembrolizumab, Any serious medical condition or laboratory abnormality or psychiatric condition or any other significant or unstable concurrent medical illness (in the opinion of the Investigator) would preclude protocol adherence or would make the safety of the study drug difficult to assess, Known or suspected SARS-CoV-2 infection, unless patient tests negative for SARS-CoV-2 within the Screening period, History of solid organ transplant or bone marrow transplant, Prior CAR-T or allogeneic cellular therapy, Ongoing systemic immunosuppressive therapy. Neo-5171 is currently in the discovery development stage. Concurrent therapy with any other investigational agent, vaccine, or device. Experimental results indicate that anti-myeloma activity is mediated by expansion of cytotoxic memory CD8 T cells and a decrease in T-regulatory CD4 cells in the bone marrow. Tick the boxes of the newsletters you would like to receive. The pharmaceutical industry's most comprehensive news and information delivered every month. About NL-201NL-201 is ade novo agonist of the IL-2 and IL-15 receptors, designed to expand cancer-fighting CD8 T cells and natural killer (NK) cells without any bias toward cells expressing the alpha receptor subunit (CD25).
 Interim data is expected to be reported in the second half of 2022. R&D Expenses: Research and development expenses for the year ended 2021 increased to $39.2 million from $24.3 million for the year ended 2020. NL-CVX1 is a de novo protein that binds to the spike protein of SARS-CoV-2, the virus that causes COVID-19 and blocks infection of human cells. MediaJulie Rathbun206-769-9219[emailprotected], InvestorsSolebury TroutAlexandra Roy617-221-9197[emailprotected], Neoleukin Therapeutics Announces Initiation of Phase 1 NL-201 Trial. Patients will receive monotherapy, intravenous NL-201 and may continue treatment until disease progression. This press release contains "forward-looking" statements within the meaning of the safe harbor provisions of the U.S. Together with our published results on the preclinical activity of NL-201 against B-cell lymphoma, we believe that a clinical trial of NL-201 in patients with hematologic malignancies is warranted.
Interim data is expected to be reported in the second half of 2022. R&D Expenses: Research and development expenses for the year ended 2021 increased to $39.2 million from $24.3 million for the year ended 2020. NL-CVX1 is a de novo protein that binds to the spike protein of SARS-CoV-2, the virus that causes COVID-19 and blocks infection of human cells. MediaJulie Rathbun206-769-9219[emailprotected], InvestorsSolebury TroutAlexandra Roy617-221-9197[emailprotected], Neoleukin Therapeutics Announces Initiation of Phase 1 NL-201 Trial. Patients will receive monotherapy, intravenous NL-201 and may continue treatment until disease progression. This press release contains "forward-looking" statements within the meaning of the safe harbor provisions of the U.S. Together with our published results on the preclinical activity of NL-201 against B-cell lymphoma, we believe that a clinical trial of NL-201 in patients with hematologic malignancies is warranted. Parts 3 and 4 The primary purpose of this study is to understand the safety of NL-201 in combination with pembrolizumab when both drugs are given intravenously in patients with advanced cancer to evaluate tolerability and to identify a recommended dose and schedule for further testing. Interim data from the ongoing systemic Phase 1 trial of NL-201 is currently anticipated in 2022. These data, generated by our collaborators at the Fred Hutchinson Cancer Research Center, demonstrate robust immune effects and anti-myeloma activity in a challenging setting, said Priti Patel M.D., Chief Medical Officer of Neoleukin. Previously presented preclinical data has demonstrated the ability of NL-201 to stimulate and expand CD8+ and NK cells at low doses with minimal impact on immunosuppressive regulatory T cells. Net Loss: Net loss for the second quarter of 2021 was $15.1 million compared to a net loss of $9.7 million in the second quarter of 2020. NL-201, in combination with a set Pembrolizumab dose, testing ascending doses and two different schedules, A programmed death receptor-1 (PD-1)-blocking antibody, NL-201 in combination with Pembrolizumab in indication specific cohorts at a dose and schedule determined in Part 3, Evaluation of tolerability of NL-201 as measured by number of subjects with dose limiting toxicities (DLTs), Evaluation of tolerability of NL-201 in combination with Pembrolizumab as measured by number of subjects with dose limiting toxicities (DLTs), Rate of adverse events in patients with advanced solid tumors, Rate of adverse event grades in patients with advanced solid tumors, Based on Investigator assessment of radiographic imaging. liability biologics Prior to Avanir, Mr. Palekar served as the Chief Commercial Officer of Medivation. Management will update timing for future trials as appropriate. For more information, please visit the Neoleukin website: www.neoleukin.com. Neoleukin believes that these findings support the further evaluation of NL-201 in hematologic malignancies. Patients will then enter long-term follow-up until starting a subsequent therapy. Key drug pipeline and competitive landscape changes based on the latest clinical activity, sent every Tuesday. (Clinical Trial), A First-in-Human Phase 1 Study of NL-201 in Patients With Relapsed or Refractory Cancer, Experimental: Part 1: NL-201 Monotherapy Dose Escalation, Experimental: Part 2: NL201 Monotherapy Expansion Cohorts, Experimental: Part 3: NL-201 in Combination with Pembrolizumab Dose Escalation, Experimental: Part 4: NL-201 in Combination with Pembrolizumab Expansion Cohorts, 18 Years and older (Adult, Older Adult), Principal Investigator: Cassandra Moore, MD, Principal Investigator: Yan Xing, MD, PhD, Los Angeles, California, United States, 90095, Principal Investigator: Wanxing Chai-Ho, MD, San Diego, California, United States, 92037, Principal Investigator: Gregory Daniels, MD, PhD, University of Colorado-Cancer Center-PPDS, Principal Investigator: Theresa Medina, MD, Jacksonville, Florida, United States, 32224, Rochester, Minnesota, United States, 55905, Principal Investigator: Brian Costello, MD, Principal Investigator: Margaret Callahan, MD, PhD, Providence Cancer Center Oncology and Hematology Care Clinic, Principal Investigator: Brendan Curti, MD, University of Utah, Huntsman Cancer Institute, Salt Lake City, Utah, United States, 80045, Principal Investigator: Benjamin Maughan, MD, PharmD, Seattle, Washington, United States, 98109, Principal Investigator: Georgianna Long, MD, Principal Investigator: Anthony Joshua, MD, Olivia Newton-John Cancer Wellness & Research Centre, Principal Investigator: Andrew Weickhardt, MD, Principal Investigator: Gary Richardson, MD, Principal Investigator: Albiruni Razak, MD.